Dr. Patrick Judeinstein

Hey there, you are just reaching what you were looking for : My new webpages !
I am a CNRS senior research scientist, working in Laboratoire Léon Brillouin located at Saclay CEA/Université Paris-Saclay (located 25 kms South West of Paris, France).
I am also "invited scientist" at Laboratoire de Physique des Solides, Université Paris-Saclay
My main research interests are summarized on the following section ;
Click on links to paper if you want to get all details on specific topics.
Low-Field NMR

Single-sided permanent magnet NMR systems allow 1D proton-density profiling, diffusion measurements and relaxometry. A specific unilateral magnet designed for low-field NMR is combined with a goniometer, to achieve micrometric precision 1D profiling, as well as spatially localized relaxometry and diffusometry on thick (150 μm membrane samples.
NMR and neutron coupling

In Soft Matter field, structure and dynamics are strongly interleaved, and it is then a keypoint to measure them simultaneously to study transient physics or unstable states. To this respect, combining Neutron Small Angle Scattering and NMR allows to reach the evolution of large structure geometries and dynamics of soft and liquid phases. Proof of concept of such experimental setup has been applied to the study of a lower Critical Solution Temperature transition driven by entropy of mixing.
R. de Oliveira-Silva al., Journal of Neutron Research (2019)
Diffusion processes in homogeneous and phase separated mixtures

Diffusion processes in dynamically asymmetric binary fluid mixtures made of monodisperse polystyrene (PS) and a rodlike nematogen molecule (5CB) are studied by pulsed-field gradient spin echo NMR in the vicinity of the phase-separation/phase-dissolution temperature. The phase-separation process and the loss of mobility of polymer chains at Tg take place simultaneously evidencing the strong effect of elasticity on the sample morphology. Characteristic length scales are estimated from modelling echo attenuation curves exhibiting diffraction-like patterns, evidencing the power of PFG-NMR to grab dynamical and structural information at the μm scale.
F. Roussel et al., Soft Matter (2008)
large scale dynamic of polymer confined in AAO membrane: 1) neutron studies

A polymer melt made of a mixture hydrogenated and deuterated high molecular mass poly(ethylene oxide) (PEO) was studied in AAO membranes under contrast matching conditions. This allows to probe the dynamical behavior of a single polymer chain under nanometric confinement in using Neutron Spin-Echo (NSE) on an extremely broad space ([0.02-0.2 Å-1]) and time ([0.1-600 10-9s]) ranges. In these conditions, no influence of confinement on the polymer dynamics was measured.
large scale dynamic of polymer confined in AAO membrane: 2) PFG-NMR

To explore larger time domains ([10-100 10-3s]), we used 1H pulsed-gradient NMR to measure micrometer-scale self-diffusion of poly(butadiene) (PBu) chains along AAO channels (20 and 60 nm). We highlight the reduction of diffusivity of confined PBu which was related only to the pore diameter but not on the molecular weight (2 to 24 kg/mol). We rationalize this by a simple volume-average model for the monomeric friction coefficient, which suggests a 10-fold surface-enhanced friction on the scale of a single molecular layer
Water confined in soft matrix

NMR is a powerful tool to sort out signals corresponding to molecular fragment with different dynamics. Measuring the amount of non-frozen water amount as well as the behavior of the gel matrix as a function of temperature is a a powerful approach to determine the level of interactions between water molecules and the polymer network. We performed such experiments to characterize materials as different as polymer matrices in environmental conditions, membranes for energy applications, biopolymer gels for tissue engineering...
K. Maver et al., J. Non-Cryst. Solids (2008)
Water confined in hard matrix

Water confined in nanodomains present unexpected properties which have much implications for technological, biological or geological issues. Depending the chemical nature of the surface (hydrophobic vs. hydrophilic) and the porous geometry, fluid phase may present fully different behavior. For water entrapped in sCNT, dramatic modification of H-bond network with respect of bulk water was observed by in-situ IR spectroscopy as a function of water uptake. In hydrophilic silica matrix, NMR experiments point out also strong variations of the water molecule dynamics related to the Gibbs-Thomson shift of melting temperature.
S. Dalla Bernardina et al., J. Am. Chem. Soc. (2016)
J. Rault et al., Eur. Phys. J. B. (2013)
Water confined at interface

Monolayer of water at interfaces correspond to extreme confinement conditions and allows to reach the specific properties of water molecules bonded to a silica surface. The strong effect of frustration of the hydrogen network is then clearly highlighted. This system was studied by an armada of experimental technique (calorimetry, MIR and NIR, neutron diffraction, QENS, NMR); and water structural and dynamical transitions were observed at low temperatures (160K, 220K and 250K). These specific properties have been nicely reproduced by an analytical mean-field percolation mode taking into account large scale fluctuating HBond network. In parallel, an MD approach is developped.
J.-M. Zanotti et al., Scientific Reports (2016)
J. Puibasset et al., Molecular Simulations (2019)
Hydration processes in H+ conducting nanostructured ionomers
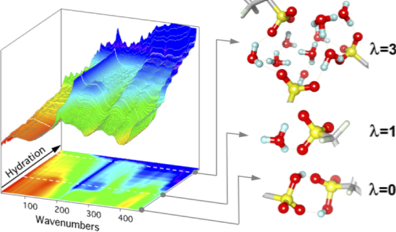
Hydrated acidic polymers are extensively studied for their potential use in fuel cells, electrolysers, ionic separation and water purification. The properties of such hydrogen- bonded nanomaterials are largely controlled by nanoconfinement and water-ions interactions. These materials possess outstanding proton conducting properties due to the interconnected H-bond network that forms inside hydrophilic channels upon water loading. In-situ far infrared (FIR) coupled to mid infrared (MIR) allows to decipher mechanisms of ionization, hydration, and intermolacular H-bonding. Influence of ionomer nanostructure and polar groups were analyzed.
CLIP : protonic ionic liquid conductivity

The structure and local organization of proton conducting ionic liquids (PCILs) obtained by reacting alkylamine with various acids were deciphered by complementary 1- and 2-D heteronuclear NMR experiments. One the one hand, PFG NMR yielded the self-diffusion coefficients of the PCIL components (and thus information on their possible concerted translational motions), while on the other hand, 13C, 1H, and 15N, 1H correlation and intermolecular Overhauser experiments gave insight into the nature of protonic species and ion-pairing behavior.
Ionic liquid multiscale dynamics

Ionic Liquids are a specific class of molecular electrolytes: they are liquids (at RT) made only of pure ions. Their unique structure leads to competition between electrostatic and van der Waals interactions and they have the propensity to self-organize in fluctuating nanometric aggregates. Strong implications on dynamical properties are then depicted, and an arsenal of spectroscopies (QENS, NSE, NMR, rheology) is then needed to probe multiscale dynamic from ps up to ms.
Anisotropic ionic liquids

Self-organization of ionic-liquid may be tuned to get anisotropic lithium electrolytes. Fast, homogeneous and uniform orientational alignment of these systems can be obtained by cooling the mixture from the isotropic melt under magnetic constraint (9.4 T). The local orientational behavior of the the different species is probed byquadrupolar NMR, including natural abundance deuterium (NAD) 2D-NMR spectroscopy. The large anisotropy of self-diffusion coefficients evidences long-range alignment of ionic slabs of these materials.
Effect of molecular architecture on the conduction process
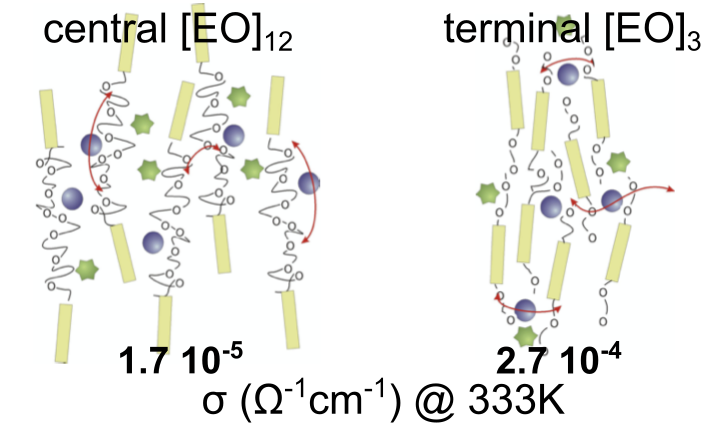
Thermotropic nematic electrolytes are obtained by dissolving lithium salts within nematogens bearing poly(ethylene oxide) (PEO) chains located either short chains at the termini or longer chains in the middle of the rigid nematogen cores. The ionic conductivity is enhanced for alkali salt/liquid crystal mixtures with short terminal PEO chains. Self-diffusion coefficients of the mobile species and the fluorine-fluorine dipolar couplings are determined by NMR, and provide insight into the ionic conductivity mechanism. These measurements point out the strong complexation of Li+ cations by PEO chains, as far as the number of EO units is larger than those necessary to complete the cation coordination shell.
P. Judeinstein et al., Adv. Mater. (2005)
Transport mechanisms in polymer electrolytes

Solid polymer electrolytes (SPEs) are generally obtained by mixing an appropriate solvating polymer with alkali salt. Ionic properties of these materials depend greatly on the solvation state of the cation and the anion as well as the ion-pairing. Dynamical properties may be probed by PFG-NMR to measure individual self-diffusion coefficients, while combination of Heteronuclear Overhauser NMR Spectroscopy (HOESY) experiments for probing through-space proximity of the different components (cation–polymer, cation–anion, anion–polymer). Potential applications and limitations of this advanced structural toolset allows to probe more complex structures such as block copolymers.
More topics to come
